The Aflac Cancer and Blood Disorders Center of Children's Healthcare of Atlanta is one of the largest pediatric cancer and blood disorders programs in the country. We care for children and young adults with a wide range of cancer and blood disorders—from the most common to those rarely seen outside of the top centers.
Current Visitor Restrictions
To protect our patients, their families and our staff, we are limiting each visitor to two parents or caregivers at the Aflac Cancer and Blood Disorders clinics.
Conditions We Treat
At the Aflac Cancer and Blood Disorders Center, our pediatric-trained experts are fully equipped to treat all cancer and blood disorders, from the most common to those rarely seen.
Our Comprehensive Programs
- Alpha thalassemia
- Anemia
- Aplastic anemia
- Beta thalassemia
- Hemochromatosis
- Hemolytic anemia
- Hemostasis and thrombosis
- Immune thrombocytopenic purpura (ITP)
- Iron deficiency anemia
- Megaloblastic anemia
- Neutropenia
- Other rare factor deficiencies
- Polycythemia
- Sickle cell disease
- Thalassemia
- Thrombocytopenia
- Von Willebrand disease
- Bone and soft tissue sarcomas
- Brain tumors
- Histiocytic and lymphoproliferative disorders
- Leukemia and lymphoma
- Neuroblastoma
- Retinoblastoma
- Wilms and other kidney tumors
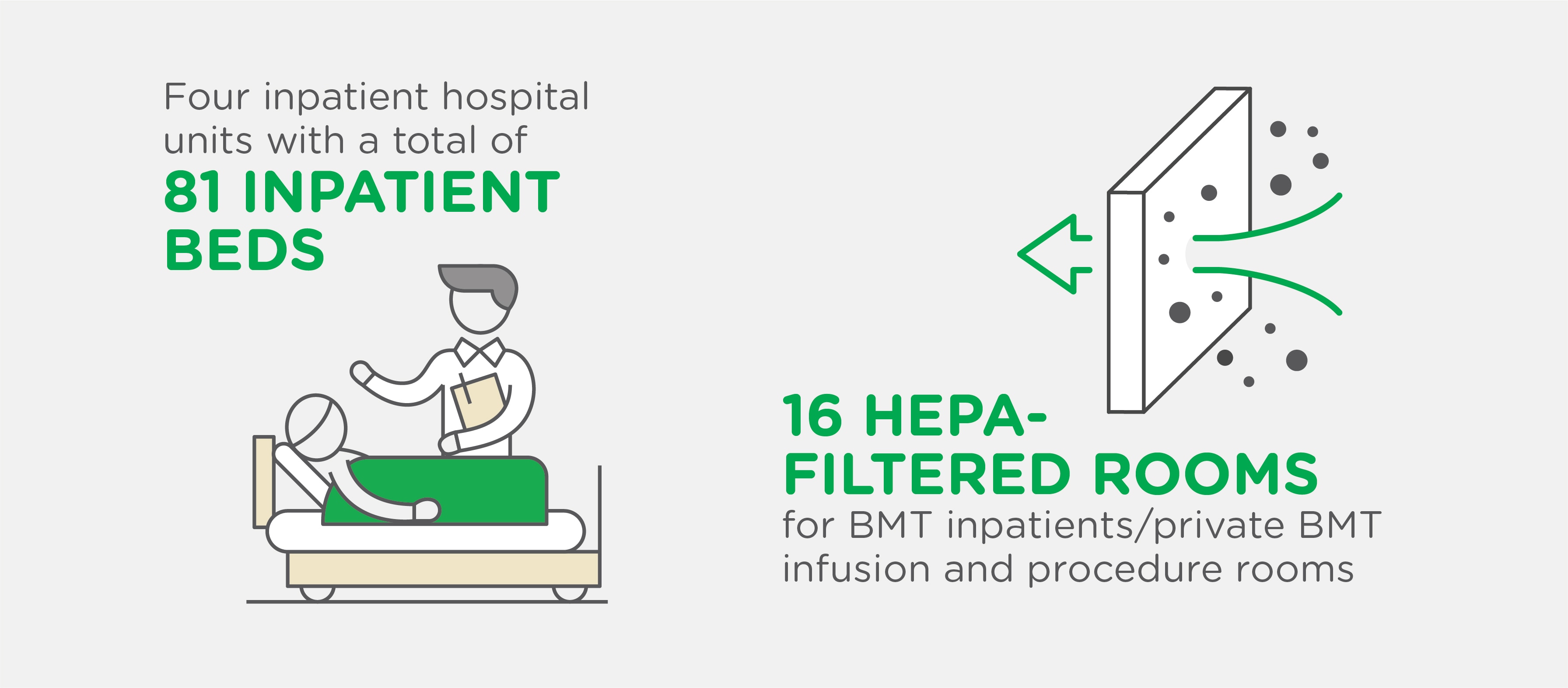
- Blood and Marrow Transplant Program
- BMT Survivor Clinic
- Bone Marrow Failure Program
- Cancer Predisposition Program
- Chronic GVHD Clinic
- Developmental Therapeutics Program
- High-Risk Leukemia and Lymphoma Program
- Immunohematology and Immune Dysregulation Program
- Infectious Disease Program
- Precision Medicine Program
- Psychology Program
- Red Blood Cell Disorders and Thrombotic Microangiopathy Program
- Supportive Care Clinic
- Survivor Program
- Young Women’s Bleeding Clinic
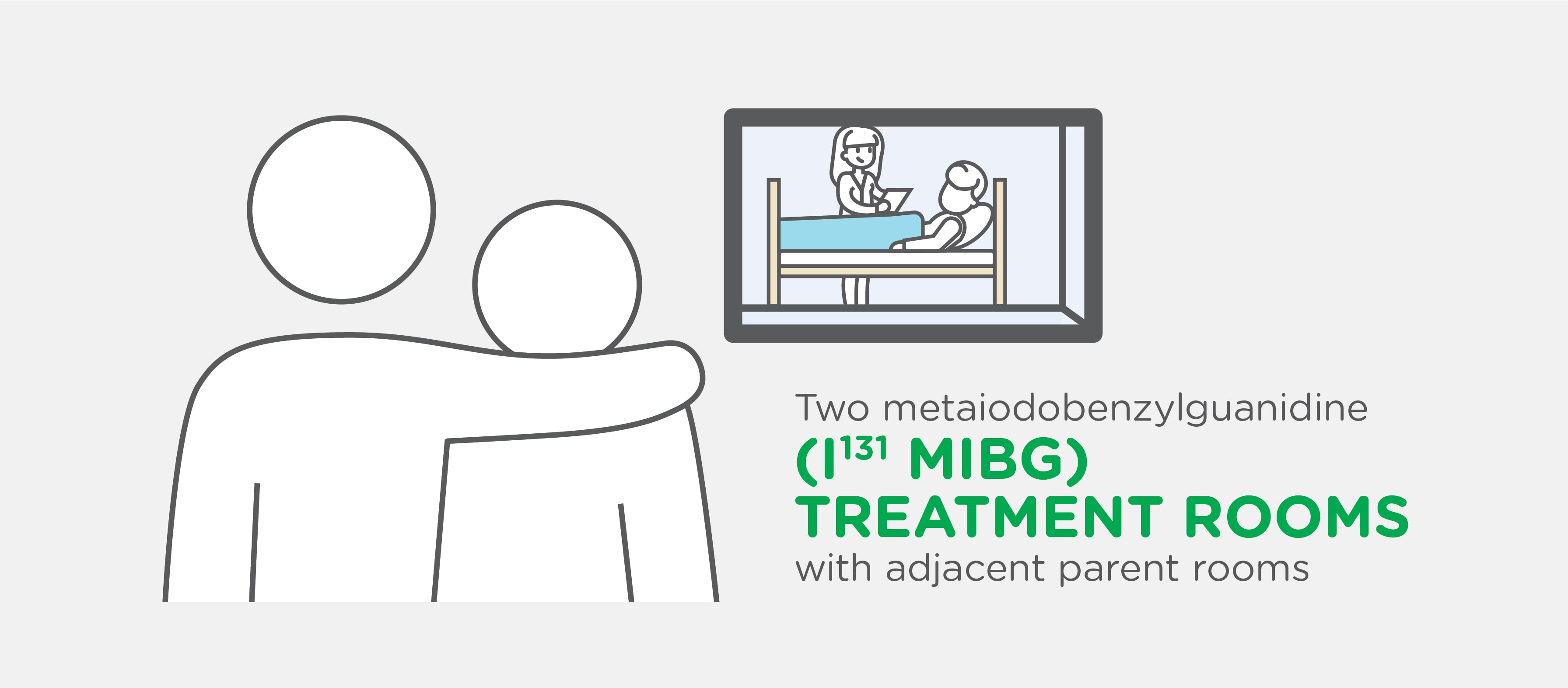
Our Advanced Center Includes:

A message from our chief
“We are extremely proud to be recognized among the top pediatric cancer programs in the nation, which is a reflection of the total dedication of our faculty, nurses and staff.”
Douglas K. Graham, MD, PhD
Chief and William G. Woods Chair
Aflac Cancer and Blood Disorders Center
The Aflac Cancer and Blood Disorders Center is committed to excellence and innovation in pediatric cancer and blood disorders research. As Georgia’s top pediatric cancer research center, we’re developing new and innovative cancer-fighting treatments right here in Atlanta. We are home to one of the largest clinical trial programs in the country—offering our patients access to over 340 clinical studies giving cutting-edge treatment to those who need it most.
The Aflac Cancer and Blood Disorders Center is uniquely positioned to leverage the vast knowledge and capabilities in Atlanta through a number of supports, endowments and partnerships. Working together, we continue to seek personalized cures for the most challenging childhood oncologic and hematologic conditions.

Make a Difference in the Lives of Kids with Cancer and Blood Disorders
Donors are the reason we’re able to treat more than 8,900 patients across all of our campuses each year and are ranked as the No. 8 in the nation among top pediatric cancer programs according to U.S News and World Report.
Get Involved
Children’s receives $20 million from Peach Bowl, Inc. to fight childhood cancer.
The overall goal of this generous Peach Bowl LegACy Fund is to help ensure that high-priority new treatment options can be tested, eventually leading to additional treatment options for our children.
READ MOREThe Future of Pediatric Cancer and Blood Disorders Care
Contact Us 404-785-1112